7.18: Comparison of E1 and E2 Reactions |
您所在的位置:网站首页 › favoured by › 7.18: Comparison of E1 and E2 Reactions |
7.18: Comparison of E1 and E2 Reactions
|
Comparing E1 and E2 mechanisms
When considering whether an elimination reaction is likely to occur via an E1 or E2 mechanism, we really need to consider three factors: 1) The base: strong bases favor the E2 mechanism, whereas, E1 mechanisms only require a weak base. 2) The solvent: good ionizing xolvents (polar protic) favor the E1 mechanism by stabilizing the carbocation intermediate. 3) The alkyl halide: primary alkyl halides have the only structure useful in distinguishing between the E2 and E1 pathways. Since primary carbocations do not form, only the E2 mechanism is possible. Reaction Parameter E2 E1 alkyl halide structure tertiary > secondary > primary tertiary > secondary >>>> primary nucleophile high concentration of a strong base weak base mechanism 1-step 2-step rate limiting step anti-coplanar bimolecular transition state carbocation formation rate law rate = k[R-X][Base] rate = k[R-X] stereochemisty retained configuration mixed configuration solvent not important polar proticExercises 1. Predict the dominant elimination mechanism (E1 or E2) for each reaction below. Explain your reasoning. 2. Which one of the following groups of compounds would eliminate \(\ce{HCl}\) most readily on reaction with potassium hydroxide? Explain your reasoning, draw the bond-line structure and give the IUPAC name of the product. a) \(\ce{(CH_3)_3CCl}\) \(\ce{CH_3CH_2CH_2CH_2Cl}\) \(\ce{CH_3CH(Cl)CH_2CH_3}\) b) \(\ce{(CH_3)_3CCH_2Cl}\) \(\ce{(CH_3)_2CHCH_2Cl}\)
c) 3. Specify the reaction conditions to favor the indicated elimination mechanism.
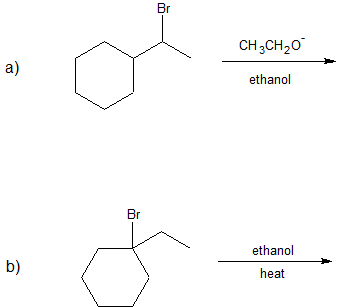
1.
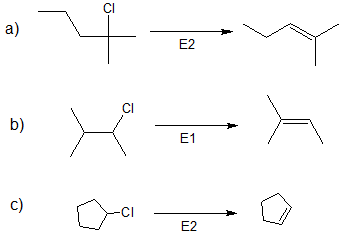
2.
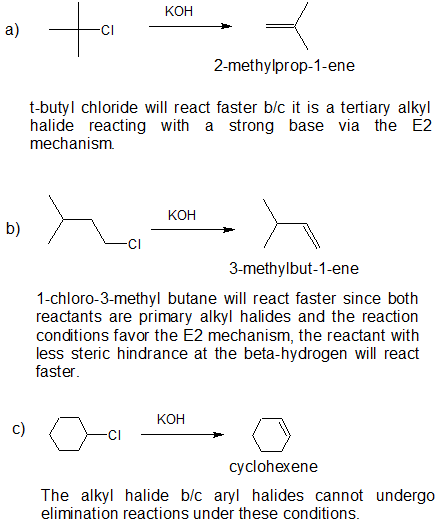
3. a) strong base, such as hydroxide, an alkoxide, or equivalent b) water or alcohol or equivalent weak base with heat c) strong base, such as hydroxide, an alkoxide, or equivalent Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris) |
【本文地址】
今日新闻 |
推荐新闻 |